- Home
-
Motors
-
Motor Controls
- Power Supplies
-
Passive Components
- Back
- Capacitors
- Circuit Breakers
- Connectors
-
Resistor
- Back
- Metal Film Resistor
- Contactors
- Current Transformer
- DIP Switch
- Electronic Ballast
- Filters
- Float Valves
- Foot Switch
- Forward Reverse Switch
- Fuse
- Hook-Up Wire
- Indicator Light
- Isolator Switch
- Junction Box
- Joystick Switch
- Knife Switch
- LED Machine Light
- Laser Module
- Locking Plugs
- Low Noise Amplifiers
- Magnetic Starter
- Micro Switch
- Over Voltage Protector
- PV Combiner Box
-
Potentiometer
- Back
- Rotary Potentiometer
- Potential Transformer
- Pressure Switch
- Push Button
- Rectifier
- Relays
- RF Attenuators
- Rocker Switch
- Rotary Switch
- Surge Protection Devices
- Tact Switch
- Terminal Block
- Timer Switch
- Toggle Switch
- Transfer Switches
-
Sensors
- Back
- Accelerometer Sensor
- Angle Sensor
- Air Quality Sensor
- Current Sensor
- Compass Sensor
- Displacement Sensor
- Encoder
- Fiber Sensor
- Flow Switch
- Float Switch
- Gyroscope Sensor
- Gas Sensor
- Inclinometer Sensor
- Ion Selective Electrode
- Load Cell
- Load Cell Transmitter
- Level Sensor
- Laser Sensor
- Limit Switch
- Light Curtain
- Magnetic Cylinder Sensor
- Noise Sensor
- Proximity Sensor
- pH Electrode
- Pressure Sensor
- PM Sensor
- Photoelectric Sensor
- Radiation Sensor
- Reference Electrode
- Rain Sensor
- Signal Isolator
- Safety Switch
- Strain Gauge
- Speed Sensor
- Soil Moisture Sensor
- Temperature Sensor
- Temperature and Humidity Sensor
- Torque Sensor
- Ultrasonic Sensor
- Voltage Sensor
- Vibration Transmitters
- Water Leakage Sensor
- Water Quality Sensor
- Wind Sensor
-
Test & Measurement
- Back
- Anemometer
- Air Quality Monitor
- Clamp Meter
- Crane Scale
- Colorimeter
- Current Transformer Tester
- Conductivity Meter
- Digital Panel Meter
- Digital Counter
- Digital Readout
- Dew Point Meter
- Digital Inclinometer
- Digital Torque Adapters
- Density Meter
- Distance Meter
- Dynamometer
- Digital Tachometer
- Digital Indicator
- Dielectric Oil Tester
- Digital Manometer
- Diameter Gauge
- Energy Meter
- Earth Resistance Tester
- Electronic Analytical Balance
- Electronic Load
- Electronic Compass
- Flow Meters
- Function Generator
- Force Gauge
- Feeler Gauges
- Gas Detectors
- Gloss Meter
- Gauss Meter
- Hipot Tester
- Hardness Tester
- Height Gauges
- Handheld Ultrasonic Homogenizer
- Infrared Thermometers
- Insulation Tester
- Linear Scale
- LCR Meter
- Laser Levels
- Lux Meter
- Land Meter
- Moisture Meter
- Multimeter
- Metal Detector
- Measuring Wheel
- Micrometers
- Measuring Tapes
- Nuclear Radiation Detector
- Network Cable Tester
- Oscilloscopes
- Optical Time Domain Reflectometer
- Oil Tank Gauge Tape
- pH Meter
- Paperless Recorder
- Pressure Gauge
- Particle Counter
- Power Meter
- Power Meter Plug
- Protractor
- Pipe Blockage Detector
- Particle Size Analyzer
- Relay Tester
- Refractometer
- Rebound Hammer
- Rebar Scanner
- Spectrophotometers
- Sound Level Meter
- Smoke Detector
- Solar Power Meter
- Surface Roughness Tester
- Signal Generator
- Stud Finder
- Temperature Controller
- Temperature Data Logger
- Thickness Gauge
- Tension Meters
- Turbidity Meter
- USB Tester
- Viscometer
- Vibration Meter
- Vernier Caliper
- Volt Amp Meter
- Water Quality Tester
- Water Leakage Detectors
-
Transmission & Actuator
- Back
- Air Filters
- Air Hose Fittings
- Angle Seat Valves
- Ball Valves
- Bearings
- Brakes and Clutches
- Butterfly Valves
- Check Valves
- Control Valves
- Diaphragm Valves
- Door Opener
- Drag Chain
- Filling Valve
- FRL Unit
- Gate Valves
- Gearbox
- Globe Valves
- Hand Valves
- Hydraulic Accumulators
- Hydraulic Actuator
- Hydraulic Cylinders
- Linear Actuators
- Linear Rail
- Linear Slide
- Needle Valves
- Pinch Valves
- Plug Valves
- Plunger Valves
- Pneumatic Cylinders
- Pneumatic Foot Pedal
- Pressure Regulator
- Pressure Relief Valves
- Pulse Valves
- Quick Connector
- Quick Exhaust Valves
- Shaft Coupling
- Shuttle Valves
- Slip Ring
- Solenoid Valves
- Steam Traps
- Strainers
- Torque Limiters
- Universal Couplings
- Vacuum Generator
- Valve Actuators
-
Pumps
- Back
- Aerator Pump
- Booster Pump
- Bilge Pump
- Centrifugal Pump
- Dosing Pump
- Diaphragm Pump
- Fire Pump
- Gear Pump
- Hydraulic Pump
- Hot Oil Pump
- Lobe Pump
- Lubrication Pump
- Magnetic Drive Pump
- Mud Pump
- Peristaltic Pump
- Piston Pump
- Pool Pump
- Rotary Hand Pump
- Screw Pump
- Self Priming Pump
- Sewage Pump
- Sliding Vane Pump
- Vacuum Pump
- Well Pump
- Wing Pump
-
Tools
- Back
- Alarm & Siren
- Beam Trolley
- Beam Clamp
- Blower
- Centrifuge Machine
- Circular Saws
- Cable Cutter
- Crimping Tool
- DC Cooling Fan
- Desoldering Tool
- Endoscope
- Electric Pressure Washer
- Foam Cutter
- Flange Spreader
- Flashlight
- Generator
- Glass Lined Reactor
- Heat Exchanger
- Hydraulic Punch
- Hoist
- Heat Gun
- Hydraulic Puller
- Hydrothermal Synthesis Reactor
- Impact Wrenches
- Inkjet Printer
- Jack
- Lifting Hook
- Labour Protection Appliance
- Magnetic Stirrer
- Magnetic Sweepers
- Portable LED Work Light
- Pipettes
- Pneumatic Screw Driver
- Plate Clamp
- Pneumatic Drill
- Pipe Bender
- Rubber Sheets
- Reciprocating Saw
- Rebar Tool
- Rotary Evaporator
- Sander
- Screw Feeder
- Soldering Tool
- Safety Mat
- Snatch Block
- Spring Balancer
- Strapping Tool
- Wire Stripper
- Winch
-
Communication & Controller
-
Industrial Equipment
- Back
- Air Compressors
- Chamfering Machines
- CNC Router Machine
- Dryer
- Dehumidifier
- Fume Extractor
- Fan Heater
- Industrial Cameras
- Industrial Vacuum Cleaner
-
Laser Machines
- Back
- Laser Marker
- Oil Mist Eliminators
- SCARA Robot
- Static Eliminator
- Steam Autoclave Sterilizer
- Tool Setter
- Ultrasonic Cleaner
- Water Chiller
- Welding Machine
- Water Purification System
- Technical Support
- How To Buy
- Contact
- Chat
-
Motors
-
Motor Controls
- Power Supplies
-
Passive Components
- Back
- Capacitors
- Circuit Breakers
- Connectors
-
Resistor
- Back
- Metal Film Resistor
- Contactors
- Current Transformer
- DIP Switch
- Electronic Ballast
- Filters
- Float Valves
- Foot Switch
- Forward Reverse Switch
- Fuse
- Hook-Up Wire
- Indicator Light
- Isolator Switch
- Junction Box
- Joystick Switch
- Knife Switch
- LED Machine Light
- Laser Module
- Locking Plugs
- Low Noise Amplifiers
- Magnetic Starter
- Micro Switch
- Over Voltage Protector
- PV Combiner Box
-
Potentiometer
- Back
- Rotary Potentiometer
- Potential Transformer
- Pressure Switch
- Push Button
- Rectifier
- Relays
- RF Attenuators
- Rocker Switch
- Rotary Switch
- Surge Protection Devices
- Tact Switch
- Terminal Block
- Timer Switch
- Toggle Switch
- Transfer Switches
-
Sensors
- Back
- Accelerometer Sensor
- Angle Sensor
- Air Quality Sensor
- Current Sensor
- Compass Sensor
- Displacement Sensor
- Encoder
- Fiber Sensor
- Flow Switch
- Float Switch
- Gyroscope Sensor
- Gas Sensor
- Inclinometer Sensor
- Ion Selective Electrode
- Load Cell
- Load Cell Transmitter
- Level Sensor
- Laser Sensor
- Limit Switch
- Light Curtain
- Magnetic Cylinder Sensor
- Noise Sensor
- Proximity Sensor
- pH Electrode
- Pressure Sensor
- PM Sensor
- Photoelectric Sensor
- Radiation Sensor
- Reference Electrode
- Rain Sensor
- Signal Isolator
- Safety Switch
- Strain Gauge
- Speed Sensor
- Soil Moisture Sensor
- Temperature Sensor
- Temperature and Humidity Sensor
- Torque Sensor
- Ultrasonic Sensor
- Voltage Sensor
- Vibration Transmitters
- Water Leakage Sensor
- Water Quality Sensor
- Wind Sensor
-
Test & Measurement
- Back
- Anemometer
- Air Quality Monitor
- Clamp Meter
- Crane Scale
- Colorimeter
- Current Transformer Tester
- Conductivity Meter
- Digital Panel Meter
- Digital Counter
- Digital Readout
- Dew Point Meter
- Digital Inclinometer
- Digital Torque Adapters
- Density Meter
- Distance Meter
- Dynamometer
- Digital Tachometer
- Digital Indicator
- Dielectric Oil Tester
- Digital Manometer
- Diameter Gauge
- Energy Meter
- Earth Resistance Tester
- Electronic Analytical Balance
- Electronic Load
- Electronic Compass
- Flow Meters
- Function Generator
- Force Gauge
- Feeler Gauges
- Gas Detectors
- Gloss Meter
- Gauss Meter
- Hipot Tester
- Hardness Tester
- Height Gauges
- Handheld Ultrasonic Homogenizer
- Infrared Thermometers
- Insulation Tester
- Linear Scale
- LCR Meter
- Laser Levels
- Lux Meter
- Land Meter
- Moisture Meter
- Multimeter
- Metal Detector
- Measuring Wheel
- Micrometers
- Measuring Tapes
- Nuclear Radiation Detector
- Network Cable Tester
- Oscilloscopes
- Optical Time Domain Reflectometer
- Oil Tank Gauge Tape
- pH Meter
- Paperless Recorder
- Pressure Gauge
- Particle Counter
- Power Meter
- Power Meter Plug
- Protractor
- Pipe Blockage Detector
- Particle Size Analyzer
- Relay Tester
- Refractometer
- Rebound Hammer
- Rebar Scanner
- Spectrophotometers
- Sound Level Meter
- Smoke Detector
- Solar Power Meter
- Surface Roughness Tester
- Signal Generator
- Stud Finder
- Temperature Controller
- Temperature Data Logger
- Thickness Gauge
- Tension Meters
- Turbidity Meter
- USB Tester
- Viscometer
- Vibration Meter
- Vernier Caliper
- Volt Amp Meter
- Water Quality Tester
- Water Leakage Detectors
-
Transmission & Actuator
- Back
- Air Filters
- Air Hose Fittings
- Angle Seat Valves
- Ball Valves
- Bearings
- Brakes and Clutches
- Butterfly Valves
- Check Valves
- Control Valves
- Diaphragm Valves
- Door Opener
- Drag Chain
- Filling Valve
- FRL Unit
- Gate Valves
- Gearbox
- Globe Valves
- Hand Valves
- Hydraulic Accumulators
- Hydraulic Actuator
- Hydraulic Cylinders
- Linear Actuators
- Linear Rail
- Linear Slide
- Needle Valves
- Pinch Valves
- Plug Valves
- Plunger Valves
- Pneumatic Cylinders
- Pneumatic Foot Pedal
- Pressure Regulator
- Pressure Relief Valves
- Pulse Valves
- Quick Connector
- Quick Exhaust Valves
- Shaft Coupling
- Shuttle Valves
- Slip Ring
- Solenoid Valves
- Steam Traps
- Strainers
- Torque Limiters
- Universal Couplings
- Vacuum Generator
- Valve Actuators
-
Pumps
- Back
- Aerator Pump
- Booster Pump
- Bilge Pump
- Centrifugal Pump
- Dosing Pump
- Diaphragm Pump
- Fire Pump
- Gear Pump
- Hydraulic Pump
- Hot Oil Pump
- Lobe Pump
- Lubrication Pump
- Magnetic Drive Pump
- Mud Pump
- Peristaltic Pump
- Piston Pump
- Pool Pump
- Rotary Hand Pump
- Screw Pump
- Self Priming Pump
- Sewage Pump
- Sliding Vane Pump
- Vacuum Pump
- Well Pump
- Wing Pump
-
Tools
- Back
- Alarm & Siren
- Beam Trolley
- Beam Clamp
- Blower
- Centrifuge Machine
- Circular Saws
- Cable Cutter
- Crimping Tool
- DC Cooling Fan
- Desoldering Tool
- Endoscope
- Electric Pressure Washer
- Foam Cutter
- Flange Spreader
- Flashlight
- Generator
- Glass Lined Reactor
- Heat Exchanger
- Hydraulic Punch
- Hoist
- Heat Gun
- Hydraulic Puller
- Hydrothermal Synthesis Reactor
- Impact Wrenches
- Inkjet Printer
- Jack
- Lifting Hook
- Labour Protection Appliance
- Magnetic Stirrer
- Magnetic Sweepers
- Portable LED Work Light
- Pipettes
- Pneumatic Screw Driver
- Plate Clamp
- Pneumatic Drill
- Pipe Bender
- Rubber Sheets
- Reciprocating Saw
- Rebar Tool
- Rotary Evaporator
- Sander
- Screw Feeder
- Soldering Tool
- Safety Mat
- Snatch Block
- Spring Balancer
- Strapping Tool
- Wire Stripper
- Winch
-
Communication & Controller
-
Industrial Equipment
- Back
- Air Compressors
- Chamfering Machines
- CNC Router Machine
- Dryer
- Dehumidifier
- Fume Extractor
- Fan Heater
- Industrial Cameras
- Industrial Vacuum Cleaner
-
Laser Machines
- Back
- Laser Marker
- Oil Mist Eliminators
- SCARA Robot
- Static Eliminator
- Steam Autoclave Sterilizer
- Tool Setter
- Ultrasonic Cleaner
- Water Chiller
- Welding Machine
- Water Purification System
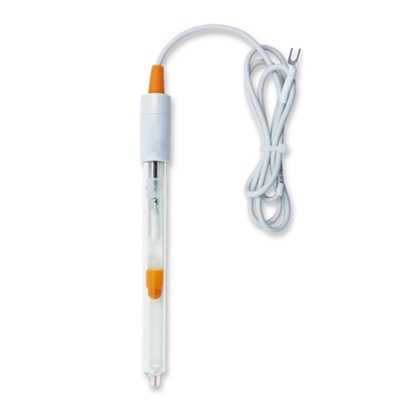
Reference Electrode
Ag/ AgCl Reference Electrode
Calomel Electrode, Hg/ Hg2Cl2 Electrode
Calomel Reference Electrode
Mercury Sulfate Reference Electrode
Saturated Calomel Reference Electrode
SCE Reference Electrode
SCE Saturated Calomel Electrode
A reference electrode is often used together with an ion selective electrode (ISE) to provide a stable reference potential to ensure measurement accuracy.
ATO provides different types of reference electrodes for your choices, they are all at favorable prices. Common types of reference electrodes include the silver/silver chloride (Ag/AgCl) electrode, saturated calomel electrode (SCE), and Mercury Sulfate (HgSO4) electrode. These reference electrodes maintain a stable potential by using a half-cell reaction with a fixed ion concentration. The Ag/AgCl electrode is widely used due to its non-toxic nature and ease of handling.
In industrial and laboratory settings, reference electrodes are essential for accurate voltage measurements and corrosion studies. They are typically combined with working and counter electrodes in a three-electrode system for electrochemical analysis.
- +1 800-585-1519 (Toll-free)
- sales(at)ato.com
- Global Shipping